“Pig hearts to be tested in humans” read a headline in the Financial Times in September 1995. The article quoted confident predictions that porcine hearts, genetically engineered to avoid rejection, would be transplanted into patients the following year — helping to ease the worldwide shortage of human organs.
More than 25 years passed before that forecast finally came true. Last month surgeons at the University of Maryland School of Medicine in Baltimore successfully replaced the failing heart of a 57-year-old man with a fresh one from a pig with 10 gene edits. Four weeks later the patient, David Bennett, continues to recover with no signs of rejection.
Xenotransplantation — replacing human organs with those from other species, typically referred to as “xeno” by scientists — has followed cycles of hope and disappointment dating back at least to 1984 when surgeons in California gave Baby Fae, born with a lethal cardiac defect, a baboon’s heart which failed after three weeks.
Now, after decades of anticipation, a new era of medical innovation could be within grasp, propelled by advances in gene editing, including Crispr technology, animal cloning and immunology. At the same time fears that transplants could transfer viruses from animals to humans, which almost killed xeno research in the late 1990s, have eased.
The Maryland cardiac team surprised the world of medicine with its announcement of a transplant of a pig’s organ into a living patient, because this did not mark the start of a formal clinical trial agreed with the US Food and Drug Administration and flagged in advance. Instead the FDA gave a one-off “emergency use authorisation”.
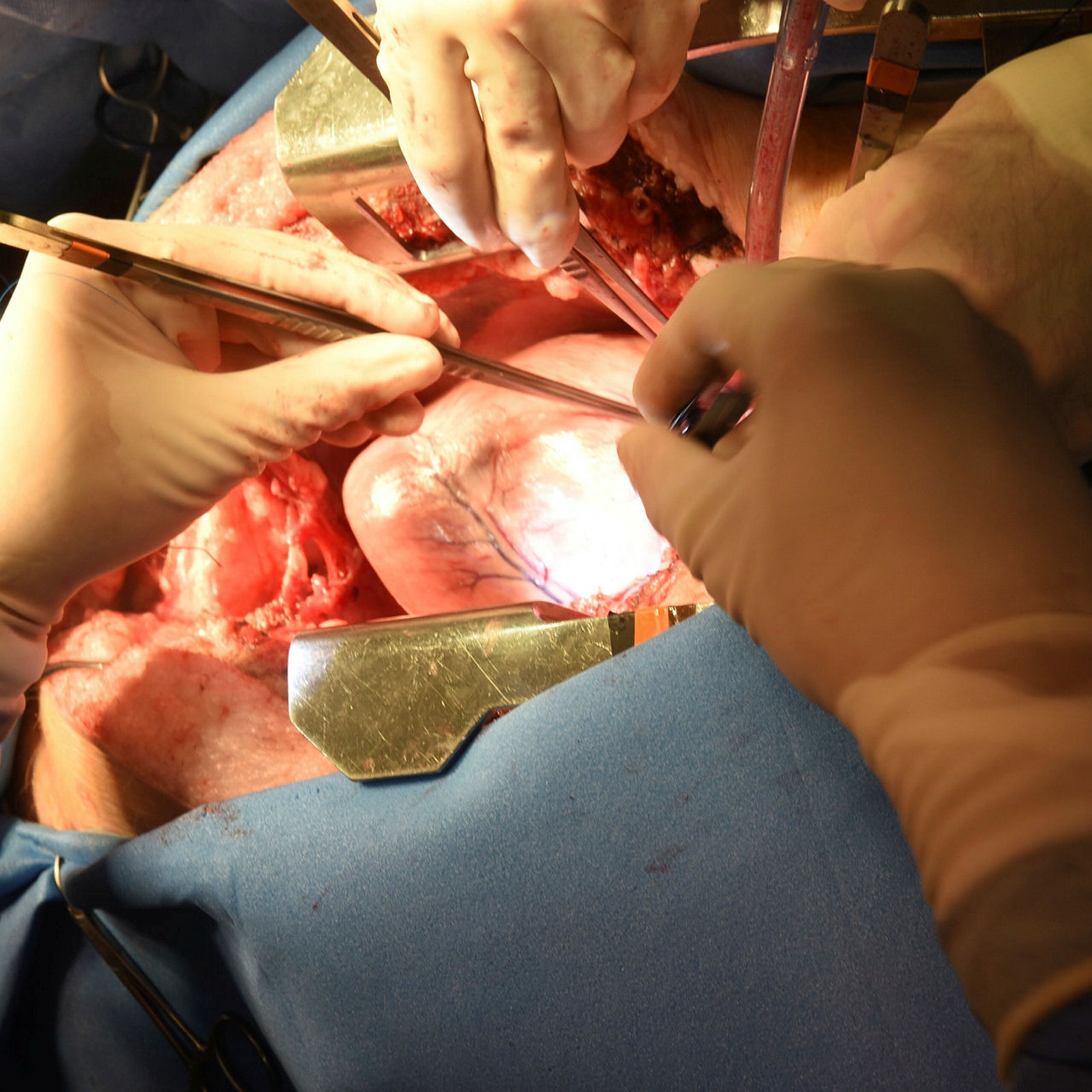
A surgeon operates on a pig prior to the removal of its heart
University of Maryland School of Medicine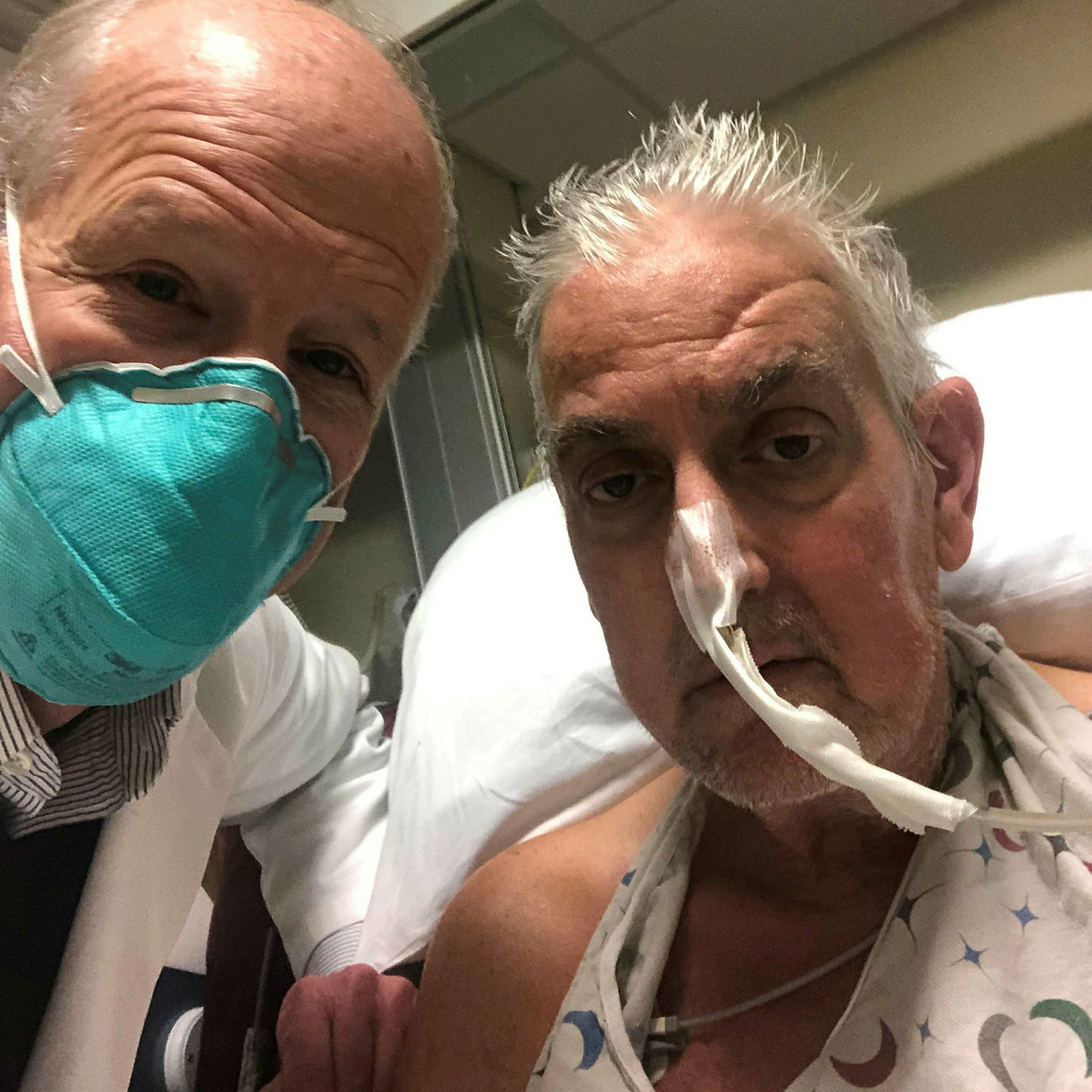
Bartley Griffith, left, was the lead surgeon for patient David Bennett, who received a pig heart at the University of Maryland Medical Center last month
University Of Maryland School Of Medicine“The FDA had never before given permission for anything like this,” says Muhammad Mohiuddin, professor of surgery and director of Maryland’s xeno programme. “The [authorisation] was granted because it was the only option for saving our patient, who was hospitalised with a life-threatening medical condition and was not eligible for a normal heart transplant.” The Maryland team received permission on New Year’s Eve and carried out the operation a week later.
Mohiuddin, who has been involved in xenotransplant research since coming to the US from Pakistan 30 years ago, says Bennett’s progress after the operation will inevitably be slow. “He was in very bad shape, having been bedridden for three months. We’ve done the equivalent of putting a new Ferrari engine in the 1960s Ford. The engine is working fine but the body will take time to adjust.
“The biggest thing we recognise in him is his will to live,” he continues. “Physicians and scientists can’t do anything for a patient without that determination.”
Xeno researchers and doctors have been carrying out pre-clinical research for several years, transplanting pig hearts and kidneys into dozens of non-human primates — mainly baboons. Now they are impatient to move on to people.
“We’re becoming better and better primate doctors, but of course that is not the goal,” says Robert Montgomery, head of the NYU Langone Transplant Institute, who himself received a new heart in 2018 from someone who had died of a heroin overdose. “I think we’re as ready as we’re going to be to move into [human trials]. It’s still a big jump, but we’re gaining confidence.”
Bennett’s “proof of concept” surgery could speed up the regulatory approval process. But there is also pressure from demographics. As populations grow older, the shortage of organs will probably grow more acute. In the US the gap between those waiting for organs and the number of transplants completed is widening. In 2002, just over 42,000 people needed transplant organs while today there are 66,216 on the waiting list.
Organs from animals that have been specifically bred for transplant purposes also have advantages in consistency and quality over those from humans, according to David Ayares, chief scientist at Revivicor, the biotech company that supplied Bennett’s new heart.
“Xenotransplants will be the solution to the organ shortage crisis,” he says. “We’ll be able to provide viable organs from young, fresh animals which can be grown to the size suited to the recipient.”
Scaling up to humans
While they wait for regulators to approve clinical trials with real patients, researchers are beginning to work with “pre-clinical human models,” or “decedents” — people who are brain-dead but whose bodily functions can be maintained for a few days through life support machines.
In three procedures last autumn, one at the University of Alabama at Birmingham (UAB) and two at New York University Langone Health, surgeons transplanted pigs’ kidneys into decedents.
Although these experiments were designed only to last for a few days before the decedents’ life support systems had to be switched off, the kidneys worked well without acute rejection, processing blood and producing urine.
The UAB team, led by Jayme Locke, operated on 57-year-old Jim Parsons who was fatally injured in a motorbike accident. He was a registered donor but his organs were not suitable for transplantation, so his family agreed to donate his body for research.
“Trying to ascertain [donated organ] function in the face of brain death is always going to be challenging,” Locke says. “Ultimately we will need to move into a phase 1 clinical trial in which we transplant these kidneys into a living human being where the environment is more favourable for kidney recovery.”

The FDA is insisting that the xeno teams build up more pre-clinical data by continuing to transplant into baboons before they start clinical trials. Locke hopes for approval by the end of this year of a phase 1 study. This might involve 10 to 20 people for whom there are no human donors, followed by an extensive phase 2 trial with a few hundred participants.
Although some of the earliest xeno work in the 20th century was done with primate donors, particularly baboons, by the 1990s researchers had settled on pigs as the best animals to use. Despite the evolutionary distance between pigs and humans — our last common ancestor lived about 80mn years ago when mammals still lived in the shadow of dinosaurs — the two species are remarkably similar in size and physiology.
A big safety issue 25 years ago was the fear of inadvertently transferring potentially fatal viruses — and particularly a group called porcine endogenous retroviruses (Pervs) — from pigs to people with the transplanted organs. Subsequent experiments have suggested that this concern was overblown and that animals reared in pathogen-free facilities do not pose a risk of infection to human recipients.
“Our animals are bred and reared behind a sterile barrier,” says Locke. “They undergo quarterly testing for zoonotic infections and specifically Pervs . . . The level of scrutiny for a pathogen-free pig facility in many ways far exceeds what is required of a human-to-human transplant.”
UK scientists played an important role in early xenotransplant research but by the early 2000s the main action had moved to the US. The leading biotech company in the field is Revivicor, based in Blacksburg, Virginia, which developed the animals behind the latest xenotransplants into humans. Revivicor was spun out of PPL Therapeutics, the Scottish animal cloning company behind Dolly the Sheep, in 2003, and was bought in 2011 by United Therapeutics.
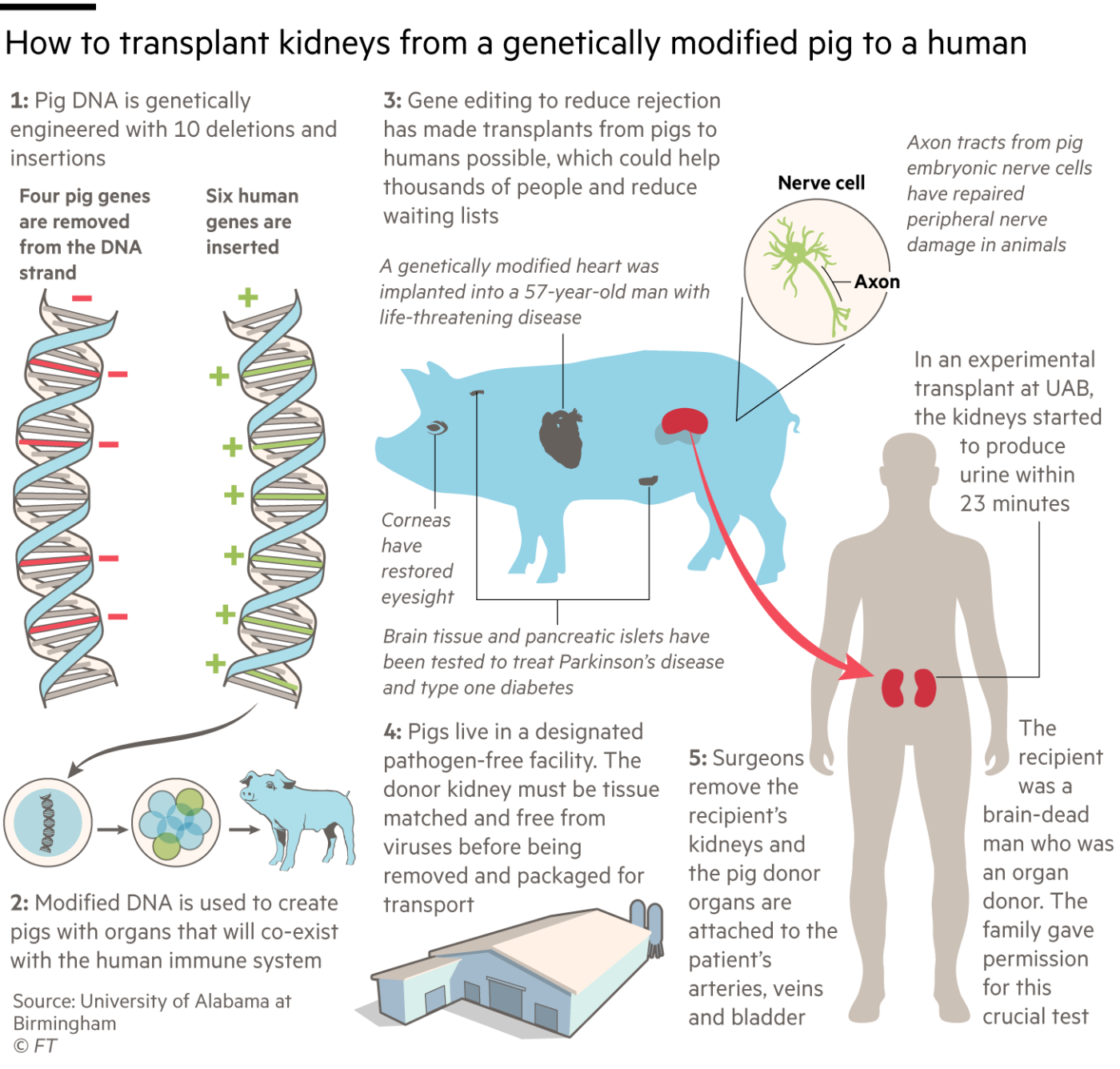
The University of Maryland and UAB teams used Revivicor pigs with 10 genetic modifications, though the company and its academic collaborators refuse to disclose how the gene editing was carried out for competitive reasons.
Four pig genes were disabled. Three of them prevent the animals adding carbohydrate molecules to cells in their organs which might cause the recipient’s body to reject the transplant. The fourth “knockout” is a pig growth hormone gene, to prevent the organ growing too large in the recipient.
The other six changes are “knock-ins” — human genes added to the pigs to prevent unwanted blood clotting, excessive inflammation and attack by antibodies. Their combined effect is to cut the risk that the human immune system will reject the porcine organs, both immediately and in the longer term.
Revivicor’s Ayares expects that pigs with the same 10 gene edits will be suitable donors for several different organs, though not all. “They are fine for heart and kidneys,” he says, “but liver and lungs have proven more challenging. For lungs we’re likely to have to tweak the genetic modifications.”
Montgomery’s NYU team is using an earlier Revivicor pig with just a single genetic modification, removing a molecule called alpha-gal, which is believed to be the most important cause of immune rejection in xenotransplants. His procedure also involves fusing the pig’s kidney with its thymus gland, which helps to control the immune system, before transplantation.
A newer xeno company is eGenesis, founded by Harvard University scientists in 2015, which collaborates with medical teams at Massachusetts General Hospital, Duke University and the University of Wisconsin. Its pigs have many of the same modifications as Revivicor’s, though one difference is that eGenesis also uses the latest Crispr gene editing technology to delete all retrovirus genes incorporated in the animal’s genome, eliminating the risk of Pervs infecting people.
“Our focus now is on getting long-term survival in non-human primate models,” says Mike Curtis, eGenesis head of R&D. “We have two [animal] recipients over 400 days post-transplant in our kidney programme . . . We are poised to be in the clinic within the next couple of years.”
If the clinical trials go well, the companies foresee no problem in increasing production to supply tens or even hundreds of thousands of animals a year for xenotransplantation. “The agriculture industry has shown us how to scale up pig production,” says Curtis.
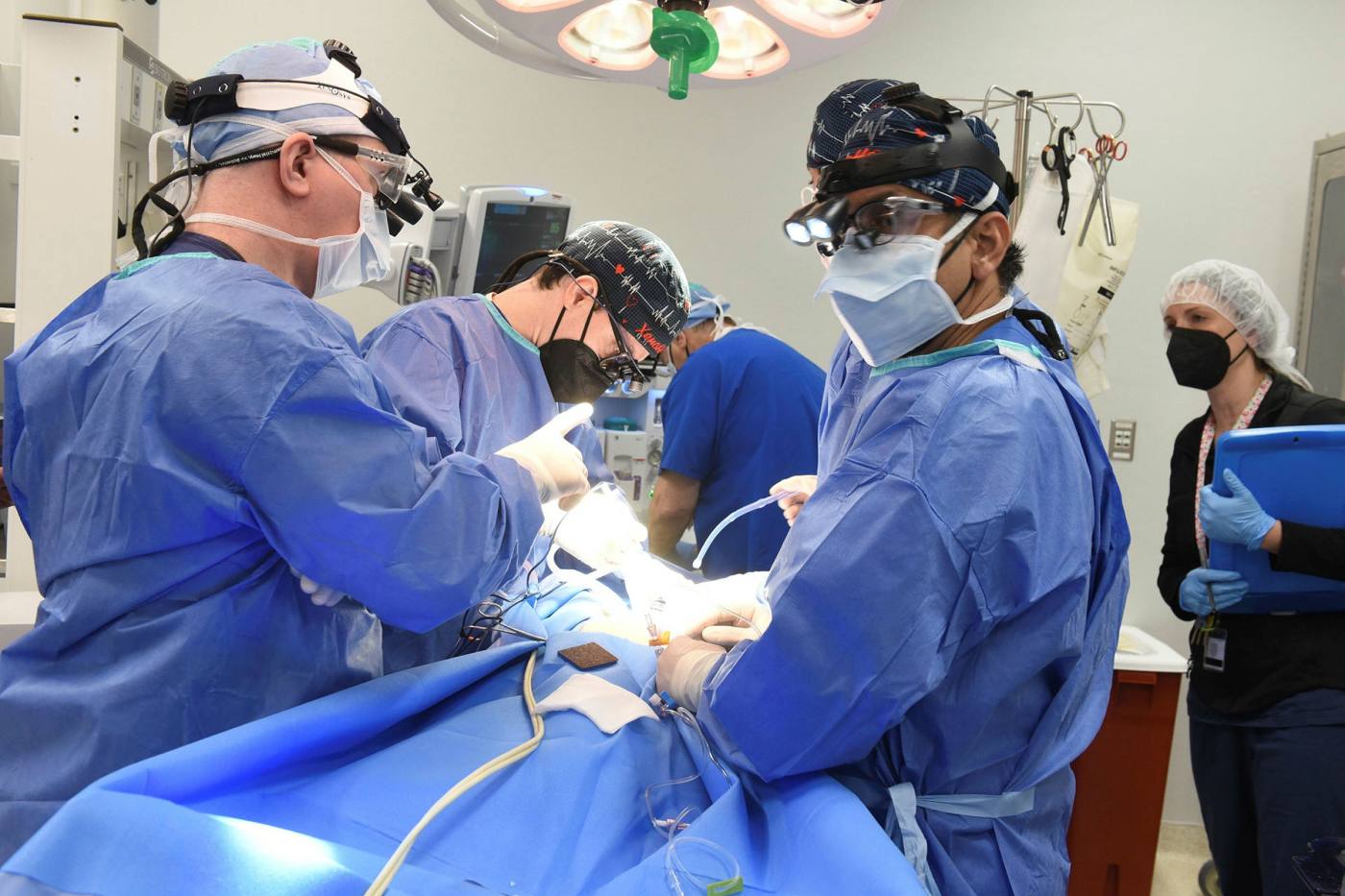
A revolution or a stop-gap?
How long pig organs will work well in people remains to be seen. At present transplanted human organs typically suffer long-term damage as a result of incompatibility with the host’s immune system. But Mohiuddin is confident that such damage can be reduced by a new generation of drugs. He is treating patients including Bennett with an experimental compound called KPL-404 which Kiniksa Pharmaceuticals is developing as a treatment for autoimmune diseases.
If xeno becomes a routine medical service, it will transform the economics of transplantation. The companies producing pig organs will make money by selling them to hospitals, while human organs are donated without payment. But other costs will fall because surgery can be scheduled in a predictable way rather than in an emergency when a donor organ becomes available.
“There’s a value proposition here beyond just the supply of organs,” says Curtis of eGenesis. “You can increase the quality, robustness and the outcomes, revolutionising how transplantation is considered,” he says. “The cost savings will be enormous if we can remove the huge burden of renal dialysis from the healthcare system.”
Meanwhile the ethical debate over breeding animals to harvest human body parts is much less intense now than it was when the first xeno experiments took place in the late 20th century. Feelings of disgust when new biological procedures are introduced — the “ugh factor” — usually fade with time, says Professor Michael Reiss of University College London, who serves on the Nuffield Council on Bioethics. That has happened with the first human-to-human transplants and with IVF babies, and seems to apply to xeno too, Reiss says.
The mainstream view among bioethicists is that xenotransplantation is acceptable as long as the animals are well cared for. “Parts of animals, including heart valves, have been used for human surgery for decades,” Reiss points out.
Religions that forbid eating pork are generally willing to accept the use of pigs to save lives, Reiss adds. “Jewish authorities decided a long time ago that it’s acceptable to use pig parts for human transplants,” he says. “Islam doesn’t have quite the same structure of authority as Judaism and Christianity, but significant Islamic ethicists have said there’s nothing that forbids it, so I imagine it would come down to individual patients.”
“The consensus [among ethicists] is that there is nothing in principle to prohibit xenotransplantation from pigs,” agrees Mohiuddin, Maryland’s xenotransplant surgeon, who is Muslim.
Mohiuddin faced another unexpected ethical objection to his pioneering operation when it turned out that the patient was sentenced to 10 years in prison for a stabbing in 1988. The family of David Bennett’s victim, who was paralysed and died prematurely, said Bennett was not a deserving recipient of the new heart. But Mohiuddin disagrees.
“We don’t check the patient’s background,” Mohiuddin says. “We choose our patients purely on clinical grounds, according to their need and suitability for the operation.”
The breakthrough in xenotransplantation offers a glimmer of hope to the many patients around the world on waiting lists for new organs. But it’s far from the only technology being pioneered to make up for a shortfall in donations.
Professor Robert Lechler of King’s College London, an immunologist whose research focuses on increasing tolerance of transplanted organs, says growing new organs from human stem cells, preferably from the recipient to avoid the risk of rejection, may be another long-term solution. An even more immediate prospect, he says, may be regenerative medicine within the body, restoring failing organs to good health so that no transplant is needed.
His colleague Mauro Giacca, professor of cardiovascular sciences at King’s, has discovered how to generate new muscle cells, myocytes, in the heart by applying short genetic control molecules called microRNA. Animal tests suggest that triggering a proliferation of myocytes after a heart attack could prevent subsequent heart failure and the need for a transplant. “The plan is to reach patients in two and a half or three years,” Giacca says.
So for now, the jury is still out on whether xenotechnology is a game-changer — or simply a stop-gap solution. “It’s quite possible that we will not need pigs’ hearts or kidneys in 10 or 20 years’ time,” says Reiss. “Xeno may be seen then as a fascinating sideline that led nowhere because it was overtaken by other approaches. Or it may be making an important contribution to healthcare.”